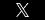According to court documents, a Massachusetts Appeals Court has ruled that a products liability lawsuit involving transvaginal mesh can move forward. The ruling reverses a lower court decision dismissing the plaintiff’s claim because she had failed to plead sufficient facts to support her claim. According to the lawsuit, the plaintiff suffered serious complications from mesh erosion, pain, and vaginal scarring. If you or a loved one has been injured by a defective product, you are encouraged to contact a local products liability attorney.
The transvaginal mesh was designed and manufactured for women who suffered from stress urinary incontinence (SUI) and pelvic organ prolapse (POP). According to Harvard Medical School, SUI and POP are caused by a weakening of the pelvic floor muscles. The transvaginal mesh is a synthetic mesh that is implanted through the vagina — transvaginally — to help support the pelvic floor muscles.
The Appeals Court judge found the plaintiff had alleged such facts, adequately detailed, so as to plausibly suggest an entitlement to relief. The plaintiff’s claim cited a 2008 FDA Public Health Notification describing thousands of complaints describing adverse effects from transvaginal mesh including pain, bleeding, infection, mesh erosion, and the recurrence of prolapse or incontinence. The plaintiff claimed that she suffered recurrent pelvic pain and had undergone multiple restorative surgeries as a result of her transviginal mesh. The judge recognized that these types of complications are among those specifically identified in the 2008 FDA notification and that the plaintiff sufficiently claimed injuries as the result of a defective product.
Defective product lawsuits are generally based on a theory of products liability. Under products liability law, manufacturers of medical devices are under a legal duty to ensure that their products are free from dangerous defects that may harm patients. There are three types of product defects that can be claimed under a products liability lawsuit: a defect in design, a defect in manufacturing, and a defective warning.
- A defect in design is a flaw in the design of the medical device. In a transvaginal mesh lawsuit, the erosion, breaking apart, and displacement may be the result of a defective design in the mesh.
- A defect in the manufacturing is a flaw in the production of a medical device that did not meet the standards of the product’s design. The same problems found in a defective design could also be attributed to a manufacturing defect where a specific mesh did not meet the standards of the products design.
- Finally, manufactures of the vaginal mesh can be liable if the manufacture failed to warn the patients about the potential problems with mesh erosion, breakage, or displacement.
In addition, in a defective product lawsuit, the manufacturer can be strictly liable, meaning that the plaintiff doesn’t have to prove fault, if the plaintiff can 1) prove that the product was defective, 2) that the defect caused the personal injury, and 3) the defect rendered the product excessively hazardous. Transvaginal mesh plaintiffs have argued that the manufacturers are strictly liable because the implants were unreasonably dangerous and the risks associated with the devices outweighed the benefits of their use.
A products liability case that severely injures a victim can be physically, emotionally, and financially difficult. An experienced products liability attorney will be able to gather evidence related to the accident and can review all the elements of your case.
If you have suffered from a defective product, we can ensure that you do not settle for less than the compensation you deserve. Local attorney, John C. Manoog III, has extensive experience handling products liability. For a free initial consultation, call the office at 888-262-6664 or reach us by email. There is always someone available to talk to you about your case.
Related Blog Posts:
Construction Worker Falls 30 Feet From Scaffolding at a Country Club in Mashpee
Massachusetts wrongful death: FDA takes on energy drink makers
 Cape Cod Injury Lawyer Blog
Cape Cod Injury Lawyer Blog